Streamline your
Pharma Labeling Process
Ensure regulatory compliance, proof artworks and leaflets, avoid labeling errors, and launch faster.
5000+ Brands















With ManageArtworks,
Streamline your workflow processes
Centralize all your assets in one place
Visualize all KPIs in one place
Track and audit all activities
Ensure error-free & compliant artworks
Reduce time-to-market
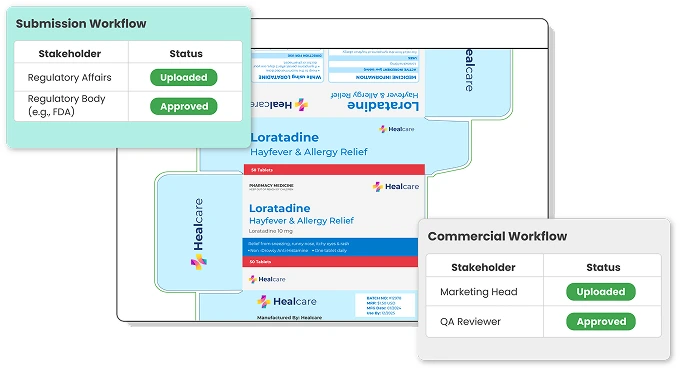
Streamline all Submission and Commercial Artwork Workflows
From initial design and regulatory approvals to commercial deployment and eventual obsolescence, the system provides a centralized hub for managing all aspects of the artwork. This not only accelerates time-to-market but also ensures compliance with regulatory standards, reduces errors, and enhances collaboration across teams.
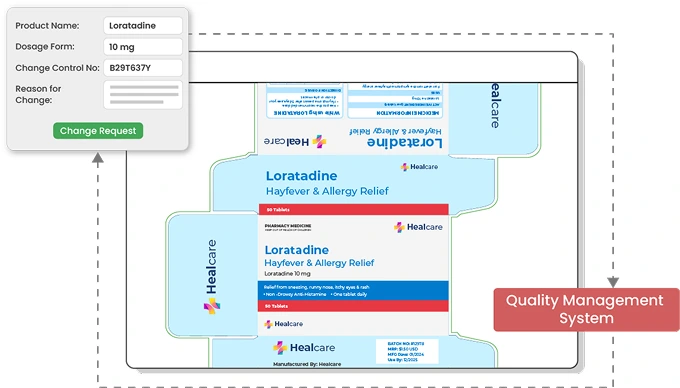
Track all Regulatory Changes
Integrate with regulatory and quality management systems to share data seamlessly. Artworks which are affected by a regulatory change with their corresponding Change Control numbers are automatically routed within ManageArtworks to respective departments for design and artwork approval.
Facilitate Text and Cutter Guide Changes
Regulatory changes can impact artworks in multiple markets, requiring text changes and translations. These changes may also necessitate cutter guide adjustments if the text doesn't fit. ManageArtworks' robust workflow system handles text and cutter guide revisions across multiple components and SKUs, with progress tracked via a central dashboard.
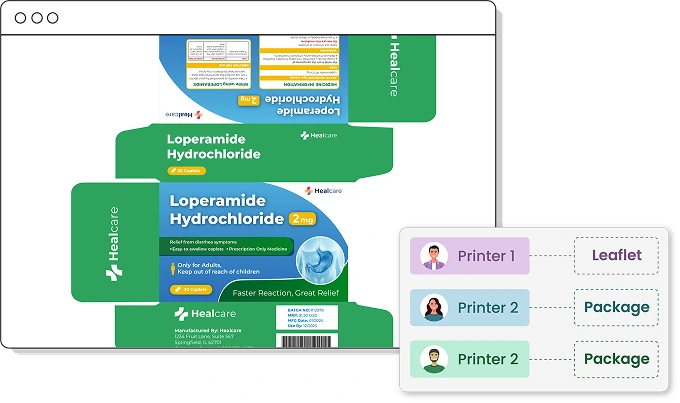
Print Proofs Approvals
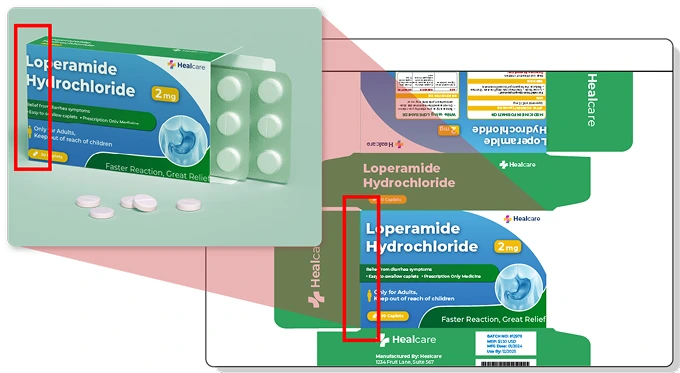
Packaging components like cartons, labels, inserts, and blister packs are often printed by different vendors—even within a single SKU. ManageArtworks lets you assign and route multiple printers for each component, ensuring precise coordination. With its built-in comparison tool, printer proofs can be instantly verified against the approved PDF to catch discrepancies early.
Link FG code and Material codes
ManageArtworks integrates with ERP systems like SAP and Oracle to retrieve FG codes, SKU descriptions, and material codes for each printed component. These codes are automatically linked to component workflows for unique identification. When an artwork component is revised, ManageArtworks supports different material codes for each artwork revision.

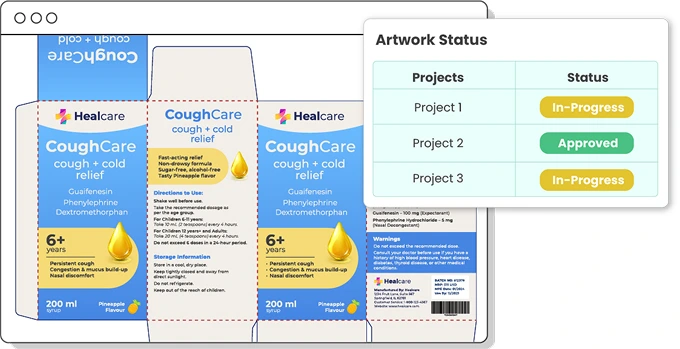
Track Artwork status
ManageArtworks assigns specific statuses to each artwork as it moves through its lifecycle, making it easy to track progress across different workflows—from initial submission to final commercial approval. In the commercial artwork workflow, once an artwork is approved, its status is updated to “Effective,” while the previous version is marked as “Superseded.” If the SKU is discontinued, the artwork status is set to "Obsoleted" or "Retired".
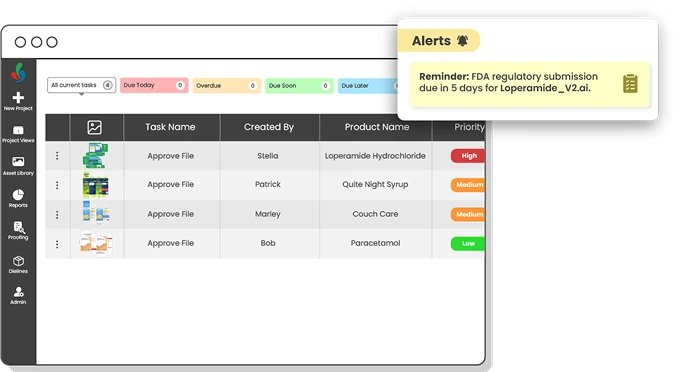
Adhere to Regulatory dates
From setting regulatory deadlines to tracking implementation and production, ManageArtworks centralizes all key dates in the artwork lifecycle. It helps teams stay compliant, plan effectively, and avoid delays—whether the required change is due in 3 months or 6. With clear visibility into the ‘Regulatory Required Date,’ ‘Effective Date,’ and ‘First Production Date,’ you stay in control from approval to execution.
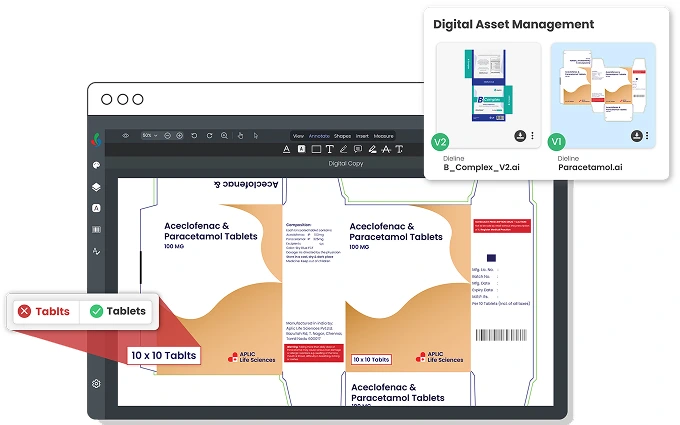
Access all your digital assets from one place
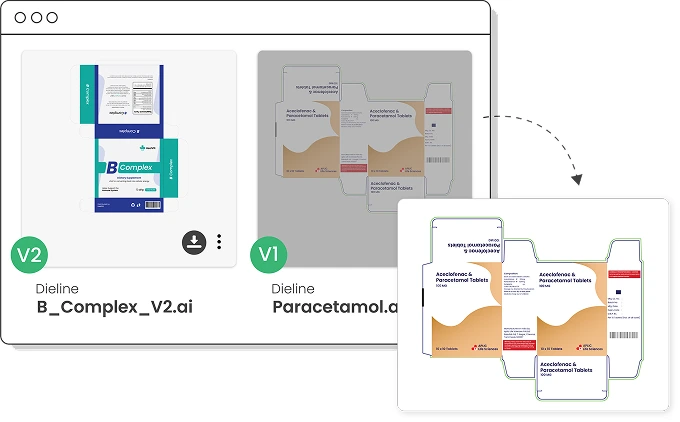
Access all approved artworks in a centralized Digital Asset Library with automatic version control.
Easily search using product metadata and always view the latest approved version.
Older revisions are auto-obsoleted based on effective dates.
Enjoy 24/7 access for seamless collaboration across teams and locations.
Swiftly analyse your data with KPI reporting
Get a clear view of all artwork projects with real-time status tracking and built-in reports.
Identify delays, first-time right rates, and iteration counts across projects.
The system automatically calculates the average artwork approval time, helping teams analyze and improve turnaround.
Older versions are automatically superseded based on effective dates, ensuring version clarity and relevance.

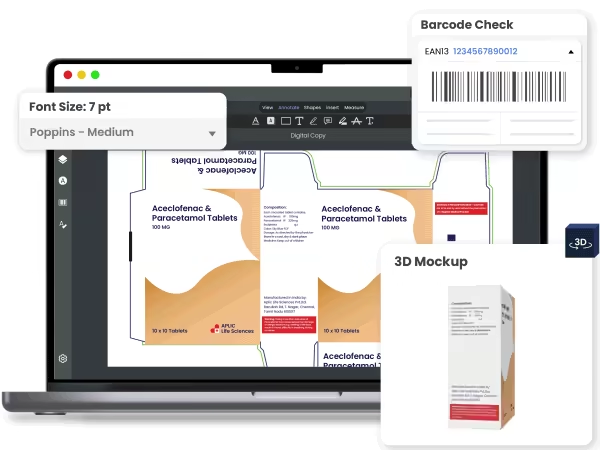
Ensure error-free artworks with AI-powered proofing

Verify color separations (CMYK and Spot), layers, and use a densitometer to check specific color locations on the artwork.
Ensure font sizes meet regulatory requirements and use the built-in spell check, which supports 25+ languages, to eliminate spelling errors.
Automatically detect and verify 1D, 2D, and Pharmacodes. The system checks against ISO 15416/15415 and GS1 standards, including scan grade, X-dimension, and Quiet Zone.
Supports Braille verification in 23 languages, decoding text in any orientation and validating dot dimensions.
Compare US leaflets or PILs across Word and PDF formats and use pixel or text comparison to highlight version changes, formatting differences, or drug facts across multiple panels.
Measure dieline dimensions and PDP areas and inspect print proofs or incoming packaging materials at the plant QC level.

Still have questions?
Can’t find the answer you’re looking for? Please chat to our friendly team.
